Ulijn Group, 2024
Welcome to the Ulijn Group website. Our lab is focused on the use of simple versions of the molecular building blocks of life to produce materials and systems with controllable structural, photonic, electronic, and biological properties. These properties open up wide-ranging applications in energy, biomedicine, environmental protection, food, cosmetics, and personal care. [Group Members and Full Biography]
We Are Recruiting!

@asrc_gc is seeking to recruit two highly motivated postdoctoral scholars to research systems-based design of biomolecular modalities and materials. Researchers who have experience or interests that bridge computation/theory and experiment are especially encouraged to consider these positions.
Come and join us in NYC! Apply here.
Adaptive peptide dispersions enable drying-induced biomolecule encapsulation
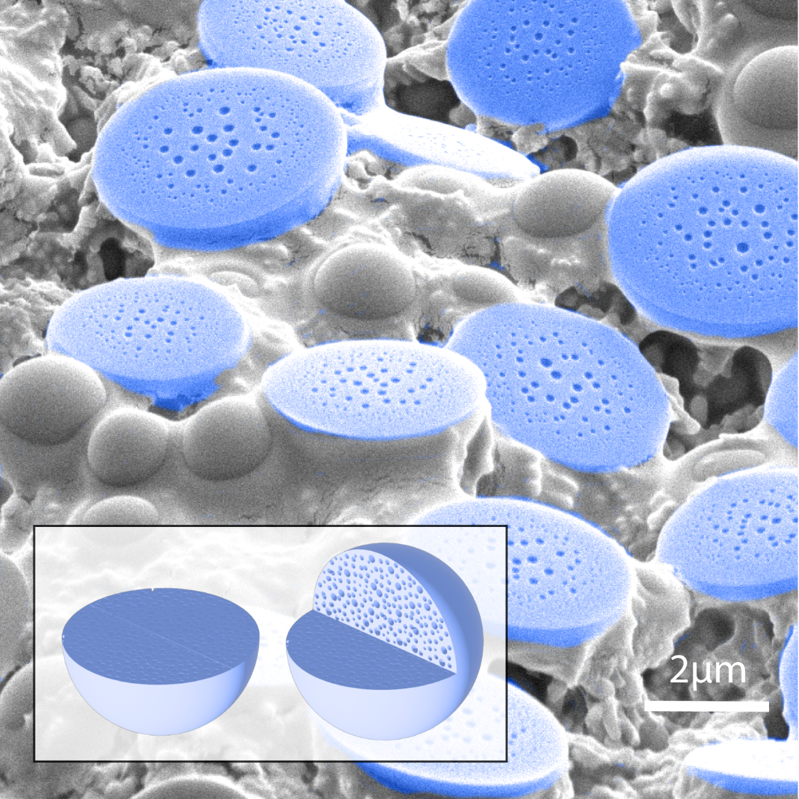
NEW YORK, August 5, 2025 — A new study from researchers at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC) reveals that extremely simple peptides can mimic a biological process that protects sensitive proteins from environmental stress. The findings, published today in Nature Materials offer a promising new approach to stabilizing biomolecules like vaccines and therapeutic proteins—potentially without the need for refrigeration. The interdisciplinary study demonstrates how short peptides—just three amino acids long—can undergo liquid-liquid phase separation through a drying process that enables the peptides to encapsulate proteins, protect them, and release them intact upon rehydration.
[More Papers]